Amylyx Pharmaceuticals Inc’s drug for amyotrophic lateral sclerosis (ALS) won the backing of external advisers from the US Food and Drug Administration on Wednesday.
The FDA panel voted 7 to 2 in favor of the oral drug, AMX0035, citing the unmet need for more treatments for the deadly neurodegenerative disease commonly known as Lou Gehrig’s disease.
Marketzone with Reuters 2022

|
|
| ||||
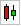
Trends Technical Analysis AMYLYX PHARMACEUTICALS, INC.
| Short term | Middle term | Long term | |
| Trends | bearish | Neutral | Neutral |
Evolution of the Income Statement
Sale Purchase | |
| Average recommendation | TO BUY |
| Number of Analysts | 5 |
| Last Closing Price | $17.90 |
| Average price target | $30.75 |
| Deviation / Average Target | 71.8% |
Officers and Directors