The Lyon-based medtech specializing in the treatment of spinal deformities has announced that it has obtained 510(k) registration from the FDA for its surgical planning software. SMAIO (Software, Machines and Adaptive Implants in Orthopaedics) will thus be able to market its software and strengthen its partnership with one of the major American players in the sector, NuVasive.

SMAIO takes a new step in the development of its software | Photo credits: SMAIO
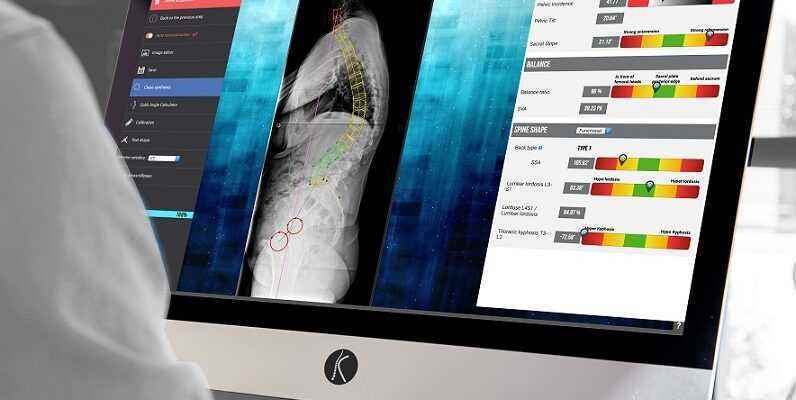
After a mixed IPO in April, the price having since lost more than 1 euro to 4.40 euros, the company specializing in the exploitation of clinical and imaging data for spine surgery is trying to find a new momentum. SMAIO (Software, Machines and Adaptive Implants in Orthopedics) has just obtained 510(k) registration from the FDA (Food and Drug Administration, American health authority) for its “Balance Analyzer 3D” surgical planning software. A ” milestone “, explains Philippe Roussouly, CEO of the French company, because this 510(k) procedure is a regulatory provision allowing the marketing of its software on the American market and therefore to generate initial income there.
In detail, KEOPS Balance Analyzer 3D is a spinal realignment planning software based on medical imaging of the patient’s spine. The company relies on a database of 100,000 surgical cases to feed its software, allowing surgeons to better understand the pathology and thus choose the best operating strategy for positioning implants and restoring the column. KEOPS is an integral part of “ the i-plan planning solution, marketed within the complete i-krontrol platform “, explains the company. In addition to planning, this platform includes solutions for controlling the execution of surgeries (i-perform) and postoperative results (i-check).
Strengthening a key partnership
This approval also constitutes a key element of the partnership concluded with NuVasive, the world’s third largest manufacturer of orthopedic implants. Through this agreement, the American company undertook to make an initial payment of $3 million one year after SMAIO’s IPO, if its platform integrating NuVasive implants obtained 510(k) registration. In total, the American manufacturer will invest 10 million dollars in the French company, half of which has already been made at the time of the IPO.
The leader of the Lyon medtech intends to rely in the years to come on this partnership, which sets ” the bases for the co-developments that the two companies have committed to carry out “. This work includes the implementation by SMAIO of an image analysis and planning assistance service for NuVasive customers. An interesting device if it succeeds, because it would generate recurring payments proportional to the analyzes carried out. This agreement also constitutes a gateway to the American market, which represents 70% of outlets in the world. An essential passage if the company counts as it has the ambition, to multiply by six its turnover, to 12 million euros, in 2025 and to post a net result in the green.
Invest