Hydrogen is considered a promising energy carrier. Directly and indirectly, it serves, among other things, for the climate-neutral propulsion of land, water and air vehicles. A persistent problem with its use is that hydrogen is a very volatile gas, and its storage has so far required some effort. It is either stored in pressure tanks at up to 700 bar or stored extremely cool in liquid form – the temperature has to be reduced to minus 253 degrees Celsius. Both ways require the use of additional energy.
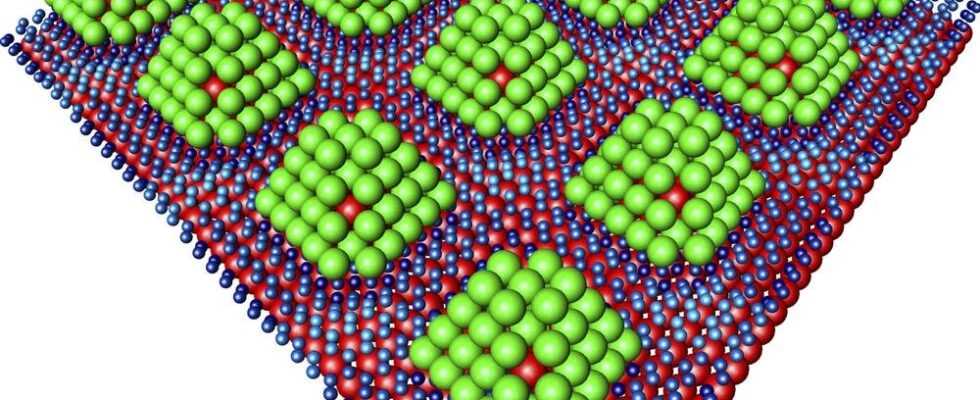
Much less effort would have to be made for storage in tiny precious metal particles. A team led by Andreas Stierle at the NanoLab of the Hamburg Research Center German Electron Synchrotron (DESY) has developed the basis for such a method. The researchers jokingly speak of “nanoprals”: they are tiny particles of palladium. It has long been known that this material sucks in hydrogen like a sponge – says Stierle, who adds: “However, until now it has been a problem to get the hydrogen out of the material again.” Therefore, his team tried it with palladium particles only about 1.2 nanometers in size – this corresponds to 0.0000012 millimeters. These tiny creatures were stabilized by a core made from the rare precious metal iridium. In addition, the researchers fixed them on a graphene foundation, an extremely thin layer of carbon. “We can anchor the palladium particles on graphene at intervals of just two and a half nanometers,” says Stierle. “The result is a regular, periodic structure.”
A slight warming is sufficient to discharge the storage tank
The equipment at DESY includes the X-ray light source PETRA III. The team was able to use this to follow what happened when the palladium particles came into contact with hydrogen: the gas essentially stuck to the surfaces – hardly anything penetrated the inside of the lumps. The researchers compare their nanoparticles with tiny pralines: In the middle there is an iridium nut, as it were, covered by a layer of marzipan made of palladium. The hydrogen is then on the outside like a chocolate coating. The advantage of this structure: A slight warming is sufficient to discharge the storage tank. The gas molecules do not have to first pave their way out of the interior, but the hydrogen quickly detaches from the particle surface.
Next, Stierle’s team wants to find out which storage densities can be achieved with their new method. However, the researchers initially put a damper on hopes of rapid use in industrial practice – there are still some challenges to be mastered. For example, the researchers are thinking about using other, more porous forms of carbon structures as a carrier material instead of graphene – something like carbon sponges. The palladium particles should then be able to be accommodated in their tiny pores in significant quantities.

Stierle’s research team, which also includes experts from the Universities of Cologne and Hamburg, published its work on October 11, 2021 in the journal “ACS Nano” of the American Chemical Society (ACS).
(psz)