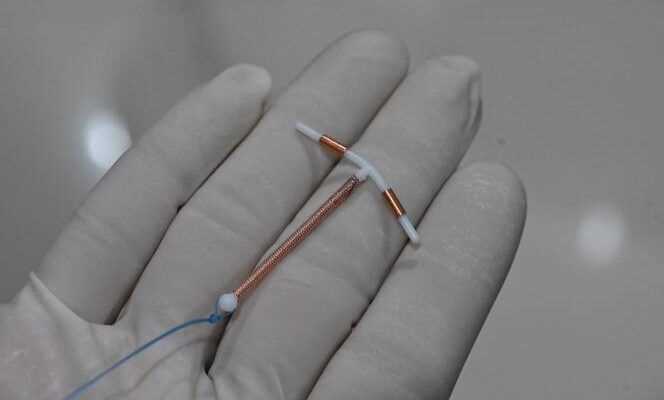
Withdrawn from sale since November 2019, the IUDs of the Ancora and Novaplus brands are still worn by thousands of women. Due in particular to a risk of spontaneous expulsion, these IUDs must be removed preventively, “Without urgency”, said Tuesday, July 27, the National Agency for Drug Safety (ANSM).
This decision concerns a maximum of 40,000 women in France, carriers of IUDs from these two brands of the Spanish manufacturer Eurogine, installed mainly in 2017 and 2018, and “Until March 2019”, specifies the ANSM. “It is a precautionary measure, without emergency, to be expected during the next consultation with your gynecologist”, his general practitioner or his midwife, explained to Agence France-Presse (AFP) Thierry Thomas, deputy director of the ANSM in charge of medical devices.
In contrast, “In case of suggestive signs” expulsion (abdominal pain, bleeding outside the period, absent or too long traction thread, pain during sexual intercourse, etc.), the women concerned must have “The reflex to go to consult immediately”, he clarified.
Ineffective IUDs
The main risk associated with this lack of stability is the ineffectiveness of the IUD as a means of contraception, which can lead to unwanted pregnancies. A recommendation had already been made to remove these IUDs after three years, as the majority of reported expulsions occurred after that time.
But the ANSM now recommends removing the intrauterine devices (IUD) concerned without waiting three years. Indeed, “Numerous reports, in particular of pregnancy or spontaneous expulsion, continue to be reported to the ANSM concerning Ancora or Novaplus models having been placed until March 2019”, explains the policeman of health products.
March 2019 corresponds to the last recall of the affected products implemented by their French distributor, so it is “The last possible date when IUDs could have been fitted” concerned, underlines Thierry Thomas. This communication is also an opportunity to remind those who have had this model for more than three years but have not yet withdrawn the recommendation.
Risk of breakage during withdrawal
Due to an increased risk of breakage during removal, healthcare professionals responsible for gynecological monitoring (gynecologists, general practitioners and midwives) are advised to proceed with caution.
“Perform a slow and constant pull by pulling the wires, then visually check the integrity of the device once removed. (…) In the event of a rupture and the persistence of a fragment inside the uterus, perform an ultrasound after the next menstruation (the residual fragment can be expelled during menstruation) ”, explains in particular the document detailing the recommendations.
Eurogine represented “Around 5% of sales of IUDs in France until 2017”, or some 20,000 devices per year, a proportion which then fell to 3% in 2018, said Thierry Thomas.